Q 2632545432
 What is the total number of orbitals associated with the principal quantum number n = 3 ?
What is the total number of orbitals associated with the principal quantum number n = 3 ?


Solution:
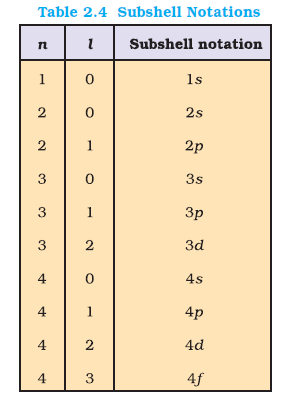
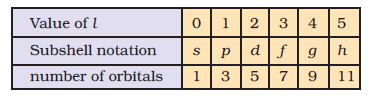
For `n = 3`, the possible values of `l` are `0`, 1 and 2. Thus there is one `3s` orbital (`n = 3, l = 0` and `m_l = 0`);
there are three `3p` orbitals (`n = 3, l = 1` and `m_l = –1, 0, +1`);
there are five `3d` orbitals (`n = 3, l = 2` and `m_l = –2, –1, 0, +1+, +2`).
Therefore, the total number of orbitals is `1+3+5 = 9`
The same value can also be obtained by using the relation; number of orbitals
`= n^2, i.e. 3^2 = 9.`
For `n = 3`, the possible values of `l` are `0`, 1 and 2. Thus there is one `3s` orbital (`n = 3, l = 0` and `m_l = 0`);
there are three `3p` orbitals (`n = 3, l = 1` and `m_l = –1, 0, +1`);
there are five `3d` orbitals (`n = 3, l = 2` and `m_l = –2, –1, 0, +1+, +2`).
Therefore, the total number of orbitals is `1+3+5 = 9`
The same value can also be obtained by using the relation; number of orbitals
`= n^2, i.e. 3^2 = 9.`